Choosing the wrong sterilization method for your medical silicone devices can be a disaster. You might face material degradation, yellowing, or even mechanical failure after just a few cycles. I want to help you understand how Ethylene Oxide (EO), Steam, and Gamma radiation specifically affect silicone performance so you can make the safest choice.
Ethylene Oxide (EO), steam autoclave, and gamma radiation each impact medical-grade silicone differently, affecting mechanical strength, color stability and dimensions. Selecting the right method requires validating these effects against your specific formulation and product design to ensure safety and longevity.
You need to know how these methods work before you can pick the right one. Let’s look at the specific mechanisms of each sterilization type and where they fit best in manufacturing.
What Are the Primary Sterilization Methods for Medical Silicone?
You need a sterilization method that kills pathogens without killing your product. If you do not understand the basic mechanisms of EO, steam, and gamma, you risk compromising the integrity of your medical devices.
The primary methods are Ethylene Oxide (EO) for heat-sensitive parts, Steam Autoclave for reusable tools, and Gamma Radiation for high-volume, single-use items. Each uses a different mechanism—chemical gas, moist heat, or ionizing energy—to achieve sterility.
I have seen many engineers struggle with this choice. At RuiYang, we often guide clients through this selection process. It is not just about killing bacteria. It is about how the silicone reacts to the process.
Ethylene Oxide (EO)
This is a gas diffusion method. We use EO for products that cannot handle high heat. The gas penetrates the packaging and the device to kill microorganisms.
- Pros: It is very gentle on materials. It operates at lower temperatures.
- Cons: It leaves residuals. You must aerate the products properly to meet ISO 10993-7 standards. It takes a long time.
Steam Autoclave
This uses moist heat and pressure. It is the standard for reusable hospital equipment.
- Pros: It is non-toxic and fast. It is inexpensive.
- Cons: High temperatures (121°C to 134°C) can change the dimensions of silicone. Moisture absorption can happen.
Gamma Radiation
This uses high-energy photons (Cobalt-60). It is common for pre-packaged, single-use items.
- Pros: It penetrates deep into sealed packages. There are no heat or moisture issues.
- Cons: It changes the molecular structure. It often causes yellowing. It can degrade mechanical properties significantly.
Here is a quick comparison table to help you visualize the differences:
| Feature | Ethylene Oxide (EO) | Steam Autoclave | Gamma Radiation |
|---|---|---|---|
| Primary Mechanism | Chemical Gas | Moist Heat & Pressure | Ionizing Energy |
| Temperature | Low (30°C – 60°C) | High (121°C – 134°C) | Ambient |
| Cycle Time | Long (includes aeration) | Short | Short to Medium |
| Best Use Case | Complex, heat-sensitive devices | Reusable surgical tools | High-volume single-use items |
How Do Different Sterilization Methods Affect Mechanical Properties and Appearance?
If you ignore the physical changes caused by sterilization, your product might fail in the field. This can lead to recalls or safety hazards for the end-user.
Sterilization impacts tensile strength, elongation, and compression set, while also causing visual changes like yellowing or haze. Gamma radiation typically causes the most significant cross-linking and discoloration, whereas steam often affects dimensional stability due to moisture absorption.
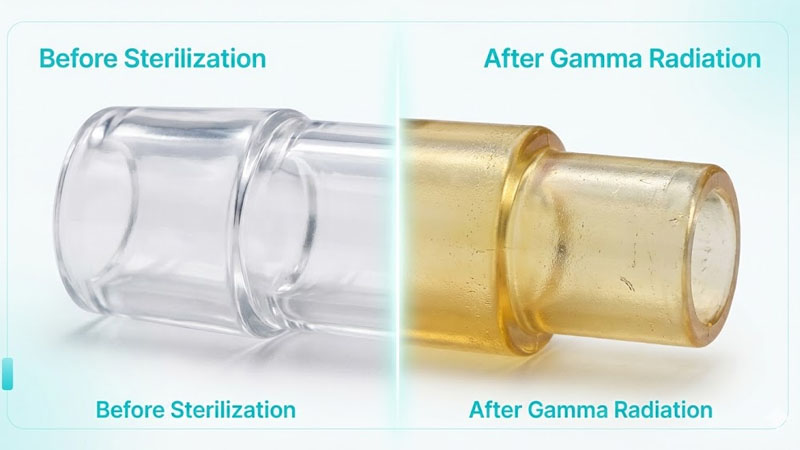
We need to dive deeper into the physical changes. I have tested many batches of silicone at RuiYang, and the results vary widely depending on the method.
Mechanical Property Changes
- Gamma Radiation: This is the most aggressive on the molecular chain. It generates free radicals. This can lead to additional cross-linking (hardening) or chain scission (softening). You will often see a decrease in elongation at break. The material becomes less stretchy and more brittle over time.
- Steam Autoclave: The heat and pressure can increase the compression set. This means if you squeeze the silicone, it might not bounce back perfectly. Repeated cycles can cause surface cracking or “crazing.”
- EO: This usually has the least impact on mechanical strength. The modulus and hardness remain relatively stable because the temperature is low.
Visual and Aesthetic Changes
Appearance matters in the medical field. A yellowed tube looks dirty or old to a doctor or patient.
- Yellowing: Gamma radiation is famous for this. The ionizing energy creates chromophores in the silicone polymer. Even “non-yellowing” grades can shift in color.
- Haze and Opacity: Steam can cause water absorption. This turns clear silicone milky or hazy. Usually, this is reversible once the moisture evaporates, but repeated cycles can make it permanent.
- Surface Defects: Steam can sometimes cause the surface to become tacky or sticky if the silicone was not cured properly during manufacturing.
Residual Effects EO leaves chemical residuals. We must strictly control these. If the aeration time is too short, the device is toxic. Steam leaves no chemical residue, but the moisture can affect electronic components inside a silicone housing.
Why Does Formulation Matter When Choosing a Sterilization Method?
Using a generic silicone formula for a specific sterilization path is a mistake. You risk unexpected reactions between additives and the sterilization medium.
Formulation sensitivity determines how silicone reacts to sterilization; pigments, reinforcing agents, and stabilizers can either protect the material or accelerate degradation. For example, platinum-cured systems generally resist yellowing better than peroxide-cured systems under gamma radiation.
I always tell my clients that the recipe matters. You cannot just pick “medical silicone” off the shelf. You need to tailor the formulation to the sterilization method.
Transparent vs. Pigmented Systems
Clear silicone shows every defect. If you use gamma radiation on clear silicone, the yellowing is obvious. However, if we add color pigments, we can sometimes mask this color shift. But be careful. Some pigments react with gamma rays and change color entirely. We have to test color stability (ΔE values) for every specific pigment.
Curing Systems: Platinum vs. Peroxide
- Platinum Cured: This is the standard for high-end medical applications. It is cleaner and has fewer by-products. It generally has better stability against radiation and heat.
- Peroxide Cured: These are cheaper but often have by-products. They are more prone to yellowing and sticky surfaces after steam sterilization. For medical use, I almost always recommend platinum-cured silicone.
Additives and Stabilizers
We can add specific stabilizers to the mix.
- Anti-yellowing agents: These help resist the color shift from gamma rays.
- Heat stabilizers: These improve the life of the product if it will go through hundreds of steam autoclave cycles.
We also have to think about catalyst residues. If there is leftover catalyst in the material, it can react during sterilization. This might cause a bad smell or increase extractables.
How Does Product Design Influence Sterilization Success?
Poor design can create failure points that sterilization aggravates. Stress concentration zones can crack after repeated sterilization cycles.
Product design features like wall thickness and geometry influence how silicone handles sterilization stress; thick sections may hold residuals longer, while thin sections may deform under heat. Addressing stress concentration zones is vital to prevent fatigue failure.
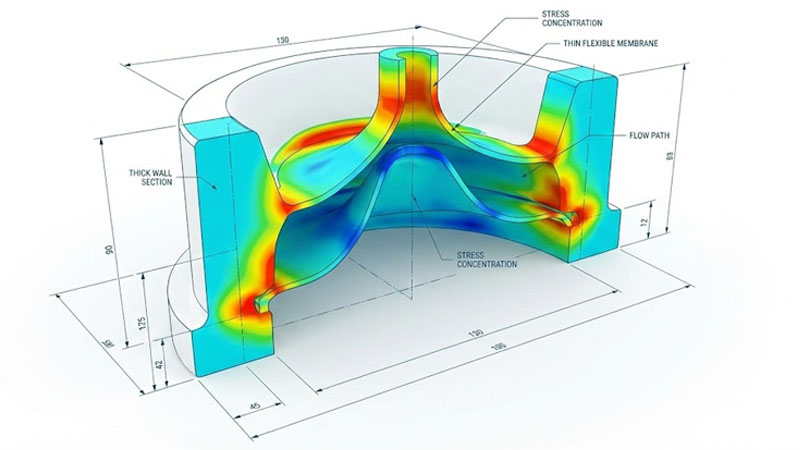
Design and material go hand in hand. I work with engineers to tweak designs before we even cut the mold.
Thin-Wall vs. Thick-Wall Sections
- EO Sterilization: If you have a very thick wall, the EO gas takes a long time to diffuse out. This increases your cycle time and cost. Thin walls aerate faster.
- Steam Sterilization: Thin walls are weak when hot. If there is a vacuum pulse in the autoclave, a thin-walled tube might collapse. Thick walls hold heat longer, which can be good for killing bugs but bad for material aging.
Stress Concentration Zones
Sharp corners are bad news. When silicone swells from heat or radiation, stress gathers at sharp corners. This is where cracks start.
- Radius: Always add a radius to internal corners.
- Undercuts: deep undercuts can trap moisture during steam sterilization. This trapped moisture breeds bacteria or degrades the silicone locally.
Multi-Material Compatibility
Many medical devices are not just silicone. They are silicone over-molded onto plastic or metal.
- Expansion Rates: Silicone expands with heat. Metal does not. If you steam sterilize a silicone-metal bond, the silicone pulls away. You need a mechanical interlock in your design, not just chemical bonding.
- Chemical Attack: EO gas might not hurt silicone, but it might attack the plastic polycarbonate part attached to it. You must check the compatibility of the whole assembly.
What Is Necessary for Validation and Accelerated Aging Testing?
Without proper validation, you are guessing at the lifespan of your product. You must prove that your device remains safe and functional after sterilization.
Validation involves cyclic sterilization testing to plot performance degradation curves and establish pass/fail criteria. You must simulate real-world usage conditions, including cleaning and storage, to accurately predict the product’s lifespan.

We cannot just test once. We have to test for the worst-case scenario.
Cyclic Test Protocol Design
If you claim your device is “reusable for 100 cycles,” we need to test it for 100 cycles. Actually, we usually test it for 110 or 120 cycles to have a safety margin.
- Step 1: Measure baseline properties (Tensile, Dimension, Color).
- Step 2: Run one sterilization cycle.
- Step 3: Clean and dry (simulating hospital use).
- Step 4: Repeat.
- Step 5: Measure properties at intervals (e.g., after 10, 50, 100 cycles).
Performance Degradation Curves
We plot the data. You will see a curve.
- Tensile Strength: It usually drops slowly over time.
- Color Change: It often changes fast in the first few cycles and then levels off.
- We set an “End of Life” point. For example, “When elongation drops by 20%, the product is expired.”
Real-World vs. Lab Testing
In the lab, we might run cycles back-to-back. In the real world, a device sits on a shelf for a week between uses. Time allows chemical reactions to continue. Real-time aging is the gold standard, but accelerated aging (using heat to simulate time) is accepted for initial submissions.
How Do We Manage Risk and Documentation for Regulatory Compliance?
Failing to document your sterilization process correctly will block your market entry. Regulatory bodies like the FDA require strict proof of safety.
Risk management requires detailed documentation of change controls, batch consistency, and biological safety assessments to meet regulatory standards like ISO 10993 and MDR. You must prove that the sterilization process does not render the device unsafe.
Paperwork is as important as the product. I have seen great products fail because the documentation was messy.
Change Control Procedures
If you switch from Gamma to EO, that is a major change. You must re-validate. Even changing the vendor who does the sterilization requires a risk assessment. You cannot simply swap methods without data.
Batch-to-Batch Consistency
Regulators want to know that Batch A reacts the same as Batch B.
- Material Certs: We keep records of every raw material batch.
- Process Parameters: We record the exact time, temperature, and dose of every sterilization run.
Labeling and User Instructions
You must tell the user what to do.
- “Non-Sterile, Sterilize before use” (for steam autoclave items).
- “Sterile, Do not re-sterilize” (for gamma items).
- If you do not label this clearly, a nurse might steam autoclave a gamma-irradiated product, causing it to fail immediately.
Regulatory Submission (510k, MDR)
For the US (FDA 510k) or Europe (MDR), you need a “Sterilization Validation Report.” This report summarizes all the testing we discussed in the previous section. It proves that the sterility assurance level (SAL) is met (usually 10^-6) and that the device still works.
Conclusion
Selecting between EO, steam, and gamma requires balancing material performance, design limits, and regulatory needs.
Would you like me to analyze your current product design and recommend the most suitable silicone formulation for your specific sterilization method?