You have probably come across the materials silicon, silica, and silicone. Although their names sound similar, their properties and uses are completely different. I will explain their differences and how they are related.
Let’s start with a simple comparison.
Key Differences at a Glance
| Property | Silicon | Silica | Silicone |
| Nature | Element | Compound | Synthetic polymer |
| Composition | Si | SiO₂ | Si–O–Si with organic groups |
| Appearance | Gray, hard solid | White powder or crystal | Soft, elastic solid |
| Source | Natural mineral | Natural mineral | Man-made |
| Common uses | Semiconductors, alloys | Glass, fillers, additives | Kitchenware, baby products |
| Flexibility | Rigid | Rigid | Flexible |
| Safety | Not for direct contact | Trace food additive | Food-grade safe for long-term use |
Silicon is a raw element. Silica is its natural oxide. Silicone is a polymer engineered from silicon chemistry.
They are related, but they behave nothing alike.
What Is Silicon? The Industrial Starting Point
Silicon is one of the most abundant elements in the Earth’s crust. You find it in sand, rocks, and minerals. In solid form, silicon is hard and brittle. It looks metallic, but it behaves like a metalloid.
From real industrial use, its key traits are clear:
- Very high melting point
- Excellent resistance to oxidation
- Predictable electrical behavior when doped
That last point is why silicon dominates electronics. Chips, solar panels, and sensors all depend on it.
Silicon itself is never soft. You would never touch it in daily products. It is only the starting material.
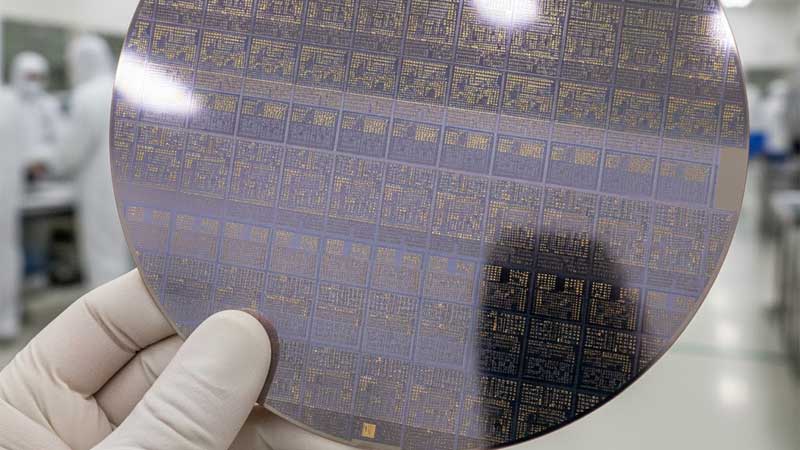
What Is Silica? Silicon in Its Natural State
Silica is silicon bonded with oxygen. Its chemical formula is SiO₂. This is what sand and quartz are made of. It is everywhere and extremely stable.
In practice, silica is:
- Hard and heat resistant
- Chemically inert
- Insoluble in water
That stability is why silica shows up in many industries.
Common real-world uses include:
- Glass and ceramic production
- Optical fibers
- Anti-caking agents in powdered foods
- Mild abrasives in toothpaste
- Fillers in rubber and electronics
Food-grade silica is used in very small amounts. It does not dissolve. It passes through the body unchanged.
From a manufacturing view, silica is also the key raw input for silicone chemistry.

What Is Silicone? The Material People Actually Use
Silicone is a synthetic polymer. It is built on a backbone of Si–O–Si bonds. This structure matters. The Si–O bond is much stronger than the carbon–carbon bonds in plastics. That is why silicone handles heat, cold, and aging so well.
At the same time, organic side groups make the material flexible. This combination is what gives silicone its unique behavior.
In real factories, the process looks like this:
- Reduce quartz sand to high-purity silicon
- Convert silicon into reactive intermediates
- Build siloxane units
- Polymerize and compound them into silicone
Different formulations give different results.
Soft baby products and firm baking mats are not the same material.
From hands-on testing, good silicone shows these traits:
- Stable from about -40°C to 230°C
- No odor during normal use
- Non-porous surface
- Long fatigue life under bending and stretching
That is why silicone works where plastics fail.
Typical applications include:
- Baking molds, spatulas, and mats
- Baby pacifiers, teethers, and bowls
- Medical tubing and seals
- Industrial gaskets and insulation

Why Silicone Works So Well in Daily Use?
Silicone is not popular by accident.
It solves real problems that plastics and rubber struggle with.
Safety in Long-Term Contact
High-quality silicone contains no BPA, no plasticizers, and no heavy metals.
Food-grade grades meet FDA and LFGB standards.
From experience, problems only appear when fillers or recycled material are added.
Pure silicone itself is stable.
Heat and Cold Resistance
Silicone does not melt like plastic.
It keeps its shape in ovens and freezers.
That reliability is critical for kitchen and baby products.
Long Service Life
Good silicone stays elastic for years.
It resists cracking, hardening, and surface breakdown.
Cheaper grades age faster.
This is usually a formulation issue, not a silicone issue.
Easy Cleaning
The surface is naturally non-stick.
Grease does not soak in.
That is a material property, not a coating.
Reuse and Sustainability
Silicone is not disposable by design.
Its durability supports reuse and waste reduction.

Common Misunderstandings I See in Real Projects
- Silicone is just another name for silicon.
- No. One is a hard element. The other is a flexible polymer.
- Silica in food equals silicone.
- No. Silica is a mineral powder used in trace amounts. Silicone is not edible.
- Silicone releases toxins when heated.
- Not true for food-grade material. Odors usually come from low-quality fillers or poor curing.
- Yellowing means it’s unsafe.
- Not necessarily. Heat, oil, and UV can cause color change without affecting safety.
Conclusion
Silicon is the source. Silica is its natural form. Silicone is the engineered material designed for real-life use.
What matters is not the name. It is the formulation, processing, and quality control behind the product.
We work directly with silicone formulations, molding processes, testing standards, and compliance requirements. From material tuning and color matching to logo and packaging integration, we support full customization based on real production experience.
If you want silicone products that perform as expected, not just look good on paper, we can help you build them right.